Published in ACS Catalysis: Electrically Driven Gaseous Ammonia Decomposition for Hydrogen Production over SiC-Mediated Catalyst without External Heating
Release time:2025-10-11 views:690
Zero-carbon hydrogen-based energy (NH3, H2) shows great application potential in automotive, shipping, renewable power storage, and industrial applications. However, the storage and transportation of green hydrogen are challenging and expensive. Ammonia, with a high hydrogen content (17.8 wt%) and ease of liquefaction, is an ideal hydrogen carrier. The use of ammonia decomposition for hydrogen production achieves on-site hydrogen generation, which can address the difficulties of hydrogen transportation and storage. In the field of transportation, ammonia fuel engines are an important technological route for fuel decarbonization. Through ammonia decomposition to produce hydrogen, ammonia-hydrogen co-combustion can resolve the ignition and combustion issues of using ammonia fuel alone, making this a key technology for ammonia fuel engines. Thus, ammonia decomposition for hydrogen production is a crucial link in the utilization of zero-carbon hydrogen-based energy. The ammonia decomposition reaction (2NH3(g) → N2(g) + 3H2(g)) can theoretically achieve a conversion rate exceeding 95% under conditions of 300℃ and 1 bar. However, due to significant kinetic resistance, traditional thermal catalysis for ammonia decomposition typically requires temperatures above 400℃ to achieve over 80% conversion efficiency. Therefore, there is an urgent need to develop new technologies for ammonia decomposition at mild conditions with high efficiency and low energy consumption.
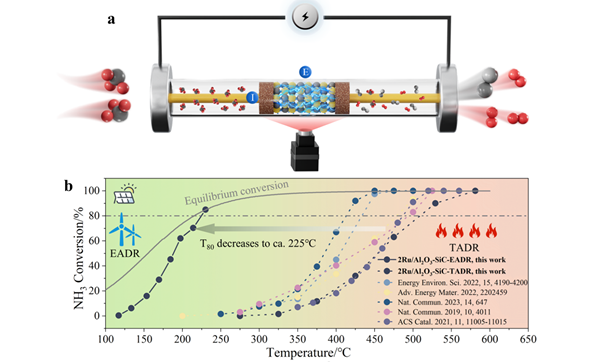
Recently, Zhang Yiran et al. from College of Smart Energy at Shanghai Jiao Tong University made significant breakthroughs in the field of energy catalysis. The research team innovatively utilized the wide bandgap characteristics and excellent thermal conductivity of third-generation semiconductor silicon carbide (SiC) to successfully construct a SiC-mediated composite catalyst with high electric field transmission capability, achieving efficient ammonia decomposition for hydrogen production under pure electric drive and without external heating for the first time globally. Research results demonstrate that under mild conditions of about 230℃, this catalyst achieved an ammonia decomposition conversion efficiency of 85%, with a hydrogen production rate of up to 0.578 mmol gcat.-1 s-1, while the energy consumption is only one-fourth that of traditional thermal catalytic methods, showing significant energy efficiency advantages. To explore the reaction mechanism in depth, the research team employed various advanced characterization techniques (including scanning/transmission electron microscopy, X-ray diffraction, X-ray photoelectron spectroscopy, and in situ desorption) combined with density functional theory (DFT) calculations to systematically reveal the reaction pathways and key rate-controlling steps of ammonia decomposition driven by the electric field. The study found that the enhanced activation of charge carriers under the influence of the electric field significantly increased the electronic density of the metal-nitrogen (metal-N) antibonding orbitals, effectively facilitating the nitrogen desorption process, thereby altering the rate-controlling mechanism of ammonia decomposition. Importantly, pioneering in situ surface reaction studies of ammonia decomposition under an electric field revealed for the first time that the transition behavior of protons (H+) in the electric field can effectively mitigate the poisoning effect of hydrogen on active sites at low temperatures, allowing the active sites to be fully exposed in the electric field environment.
This groundbreaking study proposes an electrification strategy that successfully breaks through the low-temperature kinetic limitations of traditional ammonia decomposition processes, requiring only direct current electric field input to achieve high-efficiency hydrogen production without the need for additional heating sources. This approach features significant advantages, including low energy consumption, rapid response, and compact structure. The technology not only meets the urgent demand for low-cost, portable, and rapid hydrogen production in ammonia-hydrogen conversion devices but also facilitates the direct absorption of renewable energy electricity, demonstrating broad application prospects for achieving green hydrogen production. The related work is published in the internationally renowned journal "ACS Catalysis" under the title "Electrically Driven Gaseous Ammonia Decomposition for Hydrogen Production over SiC-Mediated Catalyst without External Heating" Ph.D. student Wang Xiaochao from the School of Mechanical and Power Engineering at Shanghai Jiao Tong University is the first author, with Associate Professor Zhang Yiran from College of Smart Energy, SJTU and Professor Lin He from the School of Mechanical and Power Engineering,SJTU as co-corresponding authors. Academician Huang Zhen, founding dean of college of Smart Energy, and Chair Professor Nicolas ALONSO-VANTE provided important guidance for this work. The research was supported by funding from the National Natural Science Foundation of China and other projects.
Link:https://doi.org/10.1021/acscatal.4c06371

 WeChat
WeChat